Corti achieves EU medical device registration: Strengthening the foundation for regulated healthcare AI
Corti has reached a new milestone in healthcare AI: “Corti Assistant MD” (Medical Device) was registered as a medical device in the European Union in September. Following our UK registration earlier this year, this reinforces Corti’s role as the trusted AI foundation for HealthTech and MedTech companies across Europe.
We’re grateful to the teams and collaborators who made this possible. Your focus on safety, quality, and patient impact got us here.
What we’re announcing
- EU medical device registration: During September, Corti Assistant (medical device) was officially registered, enabling regulated deployments across the EU.
- UK medical device registration: Completed and announced earlier this year, unlocking access for NHS and other UK healthcare providers.
- Medical-grade quality system: Corti’s quality management system (QMS) is expanding throughout 2025, with external certification beginning in October.
- Infrastructure-first approach: Corti remains an API-driven platform that helps partners build safe, scalable solutions with regulatory confidence.
What this means for clinicians and users
Clinicians need tools they can trust - tools that reduce administrative burden without compromising patient safety.
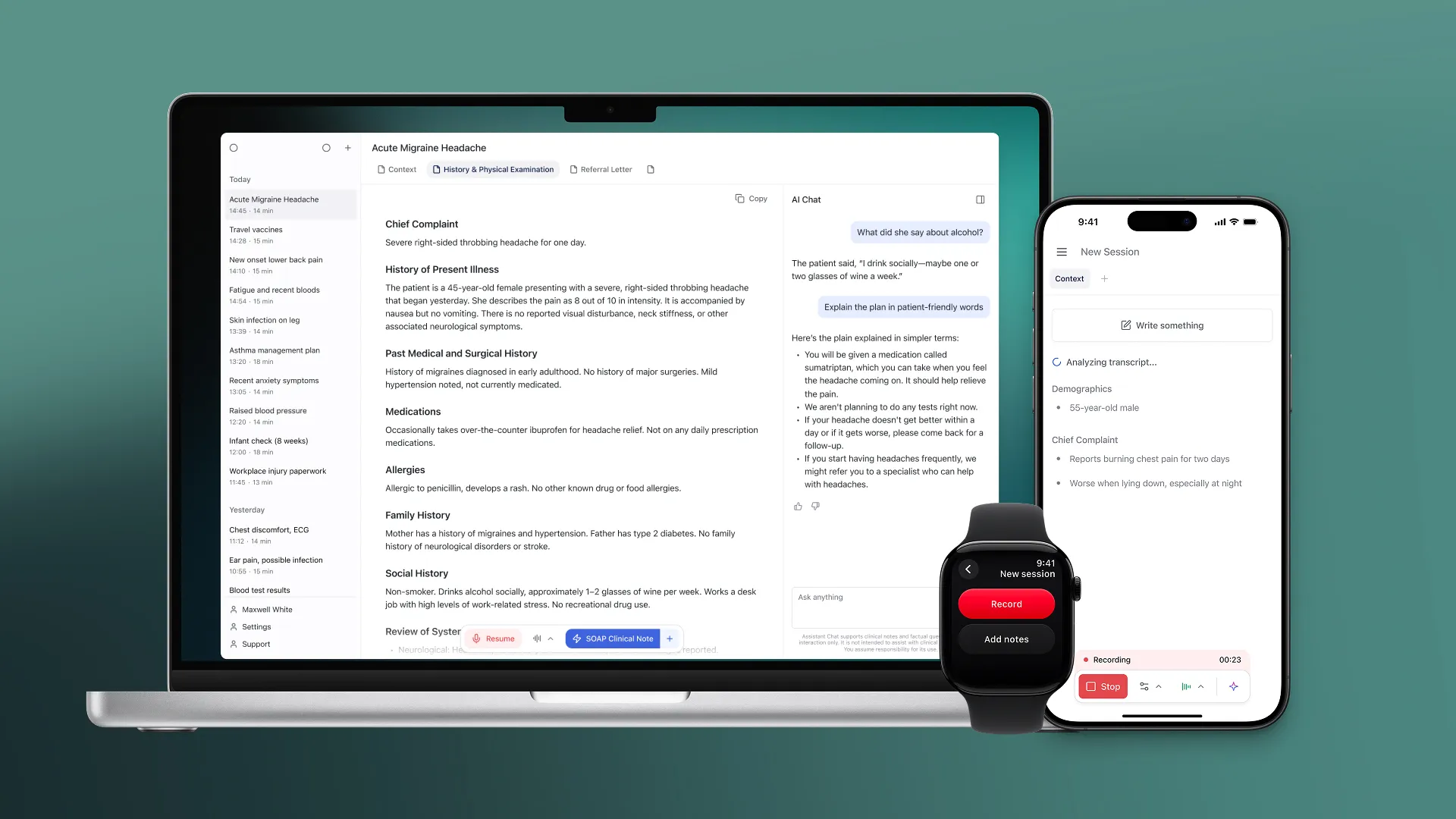
- Built for the clinical setting: Corti’s platform captures accurate, structured clinical data during patient encounters, helping clinicians stay present with patients.
- Evidence behind the product: Documented safety and performance data aligned to the stated clinical use.
- Risk management and usability: Known risks are identified, controlled, and re-assessed; workflows are tested with users.
- Quality you can audit: Development and change control happen within a regulated quality system.
- Ongoing vigilance: Real-world feedback, incident handling, and updates are part of a defined post-market process.
- Confidence in compliance: EU medical device registration (and our earlier UK registration) affirms that Corti is committed to the standards that matter in healthcare.
In short: A clinician has a tool built and maintained to medical-grade expectations, not just at launch, but throughout its lifecycle.
What this means for our partners
If you’re building on Corti’s API, you can now point to an EU-registered medical device powered by the same underlying platform. The safety and performance of Corti’s API supported products are guaranteed, based on the best practices of more than 5 ISO standards implemented.
- Medical-grade foundation: Corti’s platform supports both regulated and non regulated deployments in Europe, complementing our UK market readiness.
- Faster paths to market: We’ll provide concise partner documentation and templates so you can accelerate your own MDR journey. More info in this story.
- Flexibility by design: Whether you need a medical-device posture today or not, Corti’s API-first approach adapts to your regulatory strategy.
- The medical device registration of Corti Assistant MD does NOT create obligations for our partners and resellers of Corti Assistant or the API, yet our partners benefit from the compliant processes we’ve implemented.
Our commitment to safety & quality
Corti’s roadmap prioritizes patient safety, data security, and clinical usability. We’re continuing to mature our quality management system (QMS) and strengthen evidence across performance, risk, and real-world use—so partners and providers can adopt AI with confidence. Corti will start the external certification process of the QMS in October.
The AI platform for health and medtech
With Corti Assistant MD registered under EU MDR and UK, Corti provides a dependable, medical-grade AI platform for any healthtech and medtech company.
Health systems can adopt AI built on a regulated foundation, while partners building on Corti’s API gain a clear path to compliant deployments across Europe.
Learn more and get started
Partner overview: How to build medical-grade solutions on Corti
Have questions on this subject? info@corti.ai
Join our mission
We believe everyone should have access to medical expertise, no matter where they are.

.png)